How Is the Vsepr Theory Used to Classify Molecules
VSEPR Theory is used to predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule. The theory was first presented by Sidgwick and Powell in 1940.

Intermolecular Forces Chemistry Classroom Science Chemistry Teaching Chemistry
The vest for theory states that repulsion between the set surveillance level electrons rundown and Adam causes he sets to be oriented as far apart as possible.
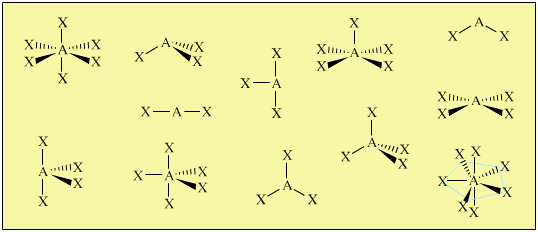
. VSEPR theory is quite successful at predicting or at least rationalizing the overall shapes of molecules. Use VSEPR theory to predict the shape of each of the following. It is a fact that electron pairs arranged around a central atom repel each other hence vesper.
The VSEPR theory or the Valence Shell Electron Pair Repulsion theory is used in chemistry to predict the geometry of molecules from the number of electron pairs surrounding the central atom. Linear In general how do intermolcular forces compare in strength with those in ionic and metallic bonding. Determines the shape of the molecules based on the number of bonded electrons and lone pairs around central atom What molecular geometry would be expected for F2 C2 O2.
Be US E pr stands for Vaillant show Electron pair proportion. It is basically a model to predict the geometry of molecules. How is VSEPR theory used to classify molecules.
See full answer below. This is based on the number of electron pairs surrounding their central atoms. The theory was first presented by Sidgwick and Powell in 1940.
410 və-SEP-ər is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. VSEPR theory classifies molecules geometrically according to the approximate arrangement of sigma bonds and lone pairs around their central atom. The VSEPR theory is a tool that is used for predicting the shape of a molecule from the electron pairs that surround the central atoms of that molecule.
Specifically VSEPR models look at the bonding and molecular geometry of organic molecules and polyatomic ions. For more details see the first two answers below and Dennis Sardellas answer to How do we determine the bond angles for molecular geometries that have lone pairs around them eg bent trigonal pyramidal seesaw etc. The VSEPR theory assumes that each atom in a molecule willachieve a geometry that.
CO H20 PCI3 QUESTIONS In the VSEPR Theory use of the general formula AXEn is another way to classify the shapes of molecules and ions A is the central atom X is the surrounding atom E lone e-pairs on A mn are integers indicating numbers of X and E respectively. There is no direct relationship between the formula of acompound and the shape of its molecules. How is the VSEPR theory used to classify molecules.
The Valence Shell Electron Pair Repulsion Model is often abbreviated as VSEPR pronounced vesper. Using this theory you can determine what shape a molecule will take in. Also best burger which is bowed.
What molecular geometry would be expected for F2 and HF. According to the VSEPR theory what molecular geometries are associated with the following types of molecules. It uses the lewis dot structures to predict the shapegeometry of a molecule.
VSEPR theory is based on the assumption that the molecule will take a shape such that electronic repulsion in the valence shell of that atom is minimized. This causes certain molecules to form geometries around a central Adam. The VSEPR theory is based on the assumption that the molecule will take a shape such that electronic repulsion in the valence shell of that atom is minimized.
Valence shell electron pair repulsion VSEPR theory can be used to predict the shape of molecules. How is the VSEPR theory used to classify molecules. The shapes of thesemolecules can be predicted from their Lewis structures howeverwith a model developed about 30 years ago known as the valence-shellelectron-pair repulsion VSEPR theory.
Thus the hypervalent species SF 6 sulfur hexafluoride with six bonding pairs is predicted and found to be a regular octahedron and PCl 5 phosphorus pentachloride with five bonding pairs is predicted and found to be a trigonal. For diatomic molecules ie those made up of two atoms the shape has to be linear. It is also named the Gillespie-Nyholm theory after its two main developers Ronald Gillespie and Ronald Nyholm.
This theory uses the concept of _________________ charges in this case the _________________ charged electrons repelling each other. VSEPR theory is used to predict the geometry of molecules. Using the VSEPR theory predict the molecular structure of each of the follo 0043.
The VSEPR theory is used to predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule. The theory was given by Sidgwick and Powell in the year 1940. How is the VSEPR theory used to classify molecules.
Valence shell electron pair repulsion VSEPR theory ˈ v ɛ s p ər v ə ˈ s ɛ p ər VESP-ər. The vsepr theory valance shell electron pair repulsion is used to determine the shape of. The VSEPR theory tells us that molecules take on regular and unique shapes because valence electrons push each other away.
Valence Shell Electron Pair Repulsion VSEPR theory is used to classify and explain molecular shapegeometry. They would both be linear. This theory is based on the repulsive interactions of electron pairs in the valence shell of an atom.
VSEPR along with atomic orbital. Table of Content What is VSEPR Theory. Valence-shell electron-pair repulsion abbreviated VSEPR and pronounced VES-per theory in which the basic principle is valence electrons around a central atom stay as far apart as possible to minimize the repulsions.
A molecule with the general formula AX4E will therefore have a _molecular shape Ohent see-saw trigonal. Use VSEPR theory to predict the geometry of cach of the following molecules. 1 Answer anor277 Nov 2 2015 Valence shell electron pair repulsion theory vesper for short classifies molecules on the basis of the number of electron pairs bonding and lone pairs associated with the central atom.
In the VSEPR Theory use of the general formula AXmEn is another way to classify the shapes of molecules and ions A is the central atom X is the surrounding atom E lone e- pairs on A m n are integers indicating numbers of X and E respectively.

Flow Chart Sn1 Sn2 E1 Or E2 Drmorrow Organic Chemistry Study Chemistry Chemistry Lessons
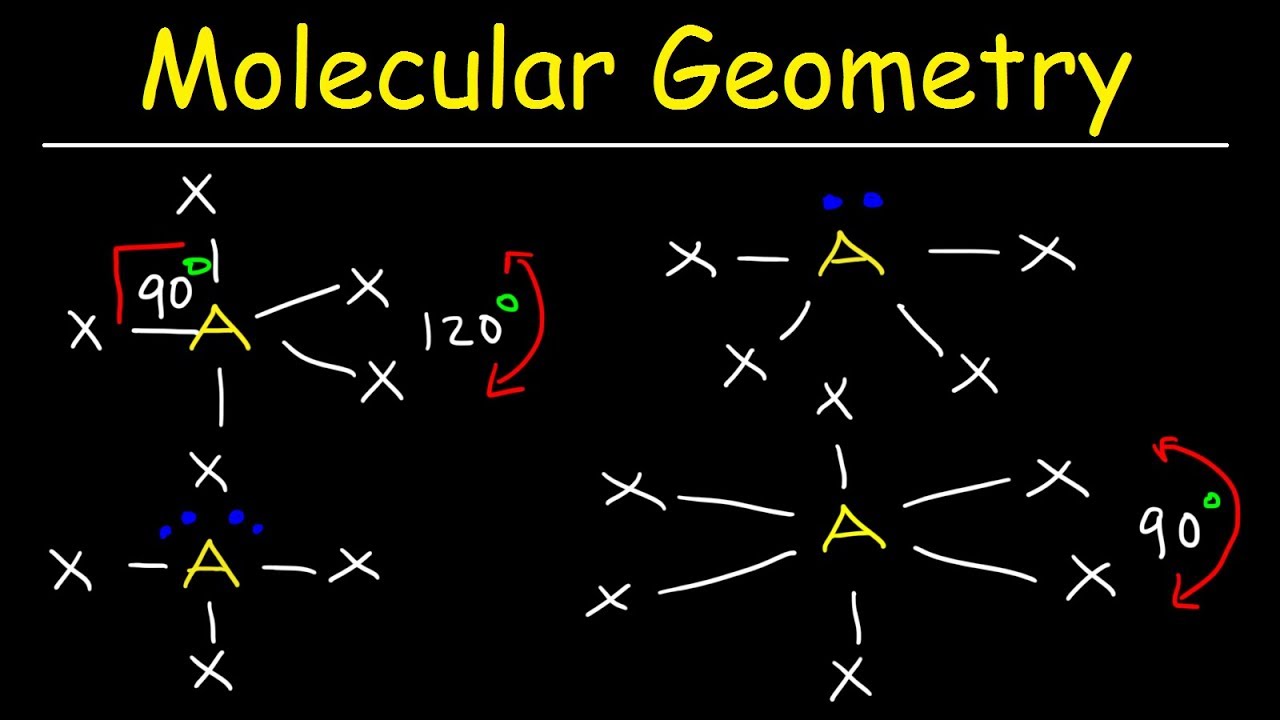
Molecular Geometry Vsepr Theory Basic Introduction Youtube
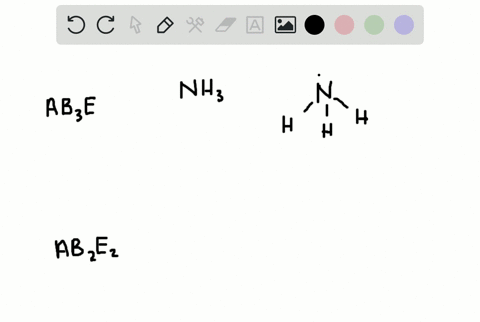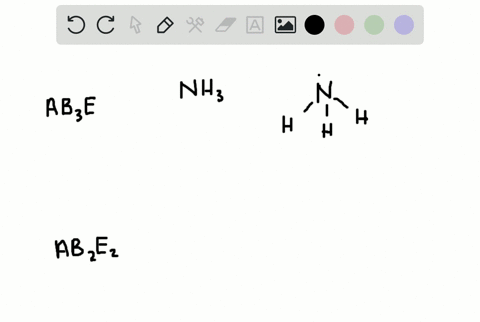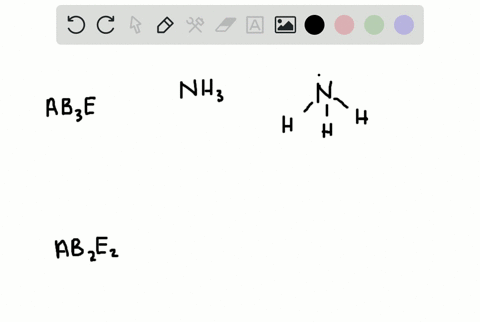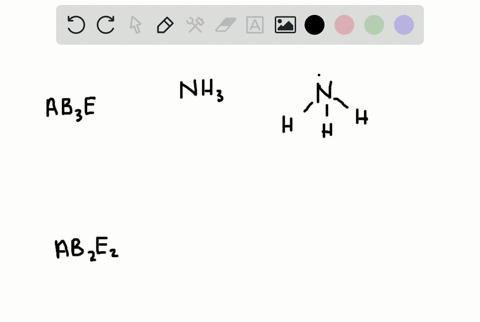
Solved A How Is The Vsepr Theory Used To Classify Molecules B What Molecular Geometry Would Be Expected For Mathrm F 2 And Mathrm Hf

Valence Shell Electron Pair Repulsion Vsepr Chemogenesis

Molecular Geometry Boundless Chemistry

Chemistry Problems Classifying Types Of Reactions Chemistry Worksheets Persuasive Writing Prompts Writing Linear Equations

We Never Know Which Lives We Influence Or When Or Why Stephen King Molecular Geometry How To Introduce Yourself Vsepr Theory

Molecular Geometry And Covalent Bonding Models

Chemical Bonding Molecular Shapes And Vsepr Theory Britannica
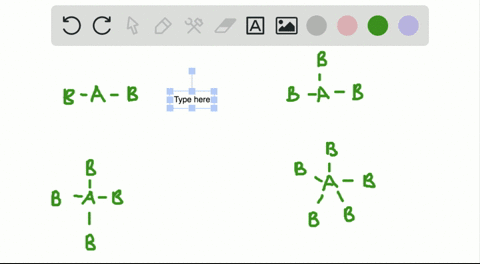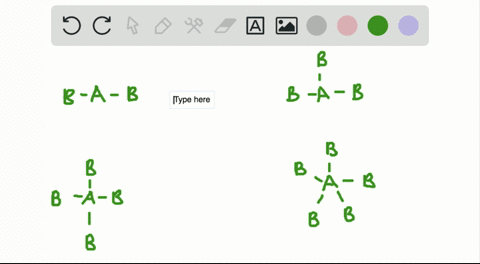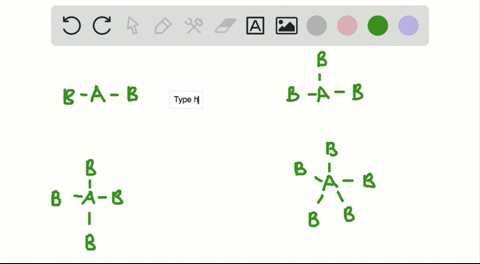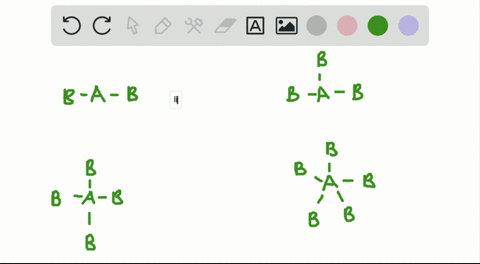
Solved A How Is The Vsepr Theory Used To Classify Molecules B What Molecular Geometry Would Be Expected For Mathrm F 2 And Mathrm Hf
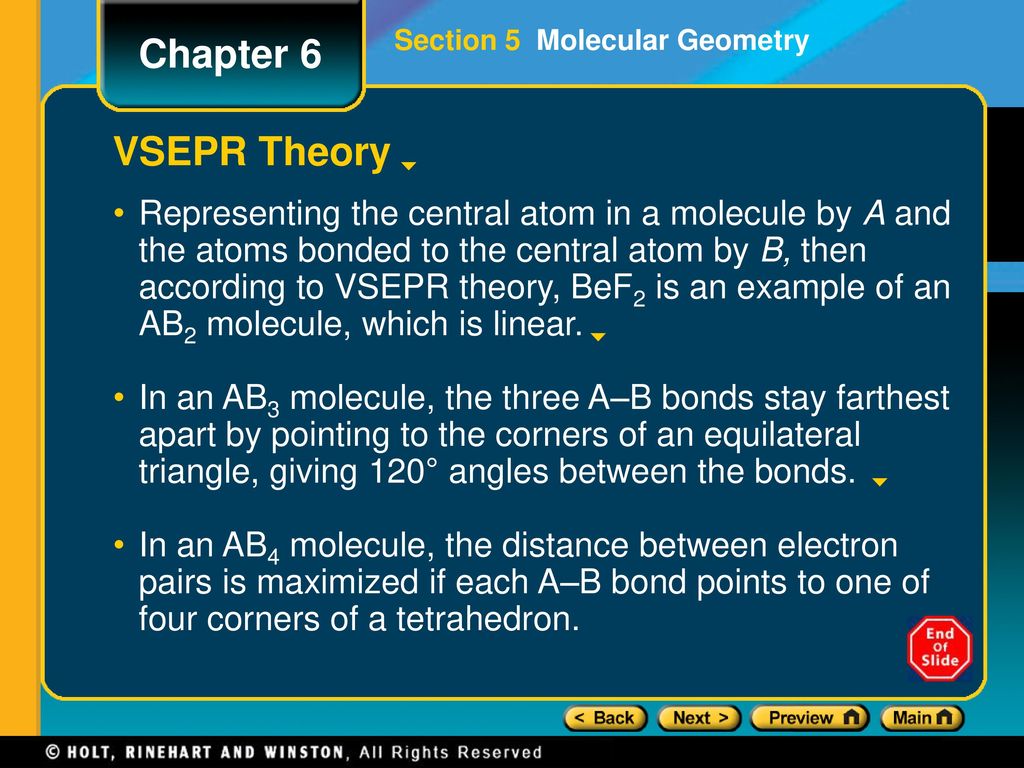
Chapter 6 Preview Objectives Molecular Geometry Vsepr Theory Ppt Download

Elements And Compounds Bundle Chemistry Activities Sharing Lessons Social Studies Resources

Molecular Geometry Boundless Chemistry

According To Vsepr Theory In Which Fashion Will The Bonds And Lone Pairs Of Electrons Are Arranged About The Central Atom In The Following Molecules Or Molecular Ions Study Com
Solved A How Is The Vsepr Theory Used To Classify Molecules B What Molecular Geometry Would Be Expected For Mathrm F 2 And Mathrm Hf




Comments
Post a Comment